From petrochemical plants to classroom benches, simple unsaturated hydrocarbons shape everyday materials and reactions. A quick scan of common members helps with picking feedstocks, understanding hazards, and spotting useful intermediates.
There are 17 Alkenes, ranging from 1,3-butadiene to trans-2-butene. For each, you’ll find below Molecular formula,Boiling point (°C),Common uses (max 15 words),Description (30-50 words), so you can compare structures, volatility, and typical applications — you’ll find below.
Which of these alkenes are most important for industry and why?
Ethylene and propylene lead because they’re primary monomers for plastics; 1,3-butadiene is key for synthetic rubber; others like trans-2-butene serve as intermediates or specialty solvents. Importance depends on scale (commodity vs. specialty), reactivity (polymerization, substitution) and supply chains.
How should I compare boiling point and uses when choosing an alkene?
Use boiling point to assess handling and storage (gaseous vs. liquid) and the listed common uses to match function—polymer feedstock, solvent, or chemical intermediate. Also weigh molecular structure (conjugation, substitution) since it affects reactivity, selectivity and safety precautions.
Alkenes
| Name | Molecular formula | Boiling point (°C) | Common uses (max 15 words) | Description (30-50 words) |
|---|---|---|---|---|
| ethene | C2H4 | -104 | Polyethylene production; fruit ripening; chemical feedstock | Ethene is a simple two-carbon alkene (C2H4), a colorless gas used to make plastics and ripen fruit commercially. Produced by cracking hydrocarbons, it’s notable as the simplest alkene and contains one carbon–carbon double bond that readily polymerizes. |
| propene | C3H6 | -48 | Polypropylene production; chemical feedstock; propylene oxide precursor | Propene is a three-carbon alkene (C3H6), a flammable gas used to make polypropylene and other chemicals. Manufactured by steam cracking or as a refinery product, it’s notable for its double bond that undergoes polymerization and many addition reactions. |
| 1-butene | C4H8 | -7 | Polyethylene comonomer; chemical intermediate; alkylation feedstock | 1-Butene (C4H8) is a four-carbon alpha-olefin used as a comonomer in polyethylene production and for synthesis. It is a colorless gas or volatile liquid, notable for its terminal double bond that reacts in polymerization and hydroformylation. |
| cis-2-butene | C4H8 | 4 | Petrochemical feedstock; solvent; alkylation | cis-2-Butene (C4H8) is a branched four-carbon alkene present as a gas or liquid and used as petrochemical feedstock. It differs stereochemically from the trans isomer, with methyl groups on the same side, affecting boiling point and reactivity. |
| trans-2-butene | C4H8 | 1 | Petrochemical feedstock; organic synthesis intermediate | trans-2-Butene (C4H8) is a geometric isomer of 2-butene used in organic synthesis and petrochemicals. The methyl groups are on opposite sides of the double bond, giving a slightly lower boiling point and distinct physical properties from the cis isomer. |
| isobutene | C4H8 | -7 | Butyl rubber production; gasoline additive precursor; chemical intermediate | Isobutene (2-methylpropene, C4H8) is a gaseous branched alkene used to make butyl rubber and methyl tert-butyl ether. Produced in refineries, it’s notable for a substituted carbon bearing the double bond, making it reactive in polymerizations. |
| 1-pentene | C5H10 | 30 | Plastic intermediate; comonomer; organic synthesis building block | 1-Pentene (C5H10) is a terminal five-carbon alkene used as an intermediate in making plastics and fine chemicals. It is a volatile liquid produced by cracking and oligomerization, and reacts readily at its double bond in addition reactions. |
| 1-hexene | C6H12 | 63 | Polyethylene comonomer; surfactant production; industrial intermediate | 1-Hexene (C6H12) is a common alpha-olefin used as comonomer in polyethylene and in surfactant synthesis. It is a colorless liquid made industrially by oligomerizing ethylene or separating from C6 fractions in refineries. |
| 1-octene | C8H16 | 121 | Polyethylene comonomer; plasticizer production; alkylation feedstock | 1-Octene (C8H16) is a higher alpha-olefin used to produce polyethylene comonomers and plasticizers. It is a clear liquid obtained by oligomerization or from wax cracking, notable for its terminal double bond allowing selective chemical modification. |
| 1-decene | C10H20 | 170 | Detergents; lubricants; polymer comonomer production | 1-Decene (C10H20) is a long-chain alpha-olefin used in detergents, lubricants, and polymer comonomers. Produced industrially by oligomerizing ethylene, it’s a liquid notable for its reactive terminal double bond used in hydroformylation and alkylation chemistry. |
| 1,3-butadiene | C4H6 | -4 | Synthetic rubber production; polymer precursor; chemical feedstock | 1,3-Butadiene (C4H6) is a conjugated diene used to make synthetic rubbers and plastics. It is a gas at room temperature, produced by cracking, notable for two double bonds that enable polymerization and Diels–Alder reactions. |
| isoprene | C5H8 | 34 | Natural and synthetic rubber production; chemical intermediate | Isoprene (2-methyl-1,3-butadiene, C5H8) is a natural diene used to make natural and synthetic rubber. Emitted by plants and produced industrially, it’s notable for two conjugated double bonds that make it highly reactive in polymer chemistry. |
| styrene | C8H8 | 145 | Polystyrene production; resins; polymer precursor | Styrene (C8H8) is a vinyl-substituted aromatic monomer used to make polystyrene plastics and resins. Produced from ethylbenzene, it’s a liquid notable for a vinyl (C=C) group attached to a benzene ring that readily polymerizes. |
| cyclohexene | C6H10 | 83 | Solvent; chemical intermediate; organic synthesis | Cyclohexene (C6H10) is a six-membered cyclic alkene used as a solvent and chemical intermediate. Found in petroleum and made by hydrogenation routes, it’s notable for one non-aromatic double bond in a ring that undergoes addition reactions. |
| cyclopentene | C5H8 | 49 | Organic synthesis intermediate; material precursor | Cyclopentene (C5H8) is a five-membered cyclic alkene used in organic synthesis and material precursors. It is a volatile liquid produced from petroleum fractions, notable for its ring double bond which is reactive in hydrogenation and polymer chemistry. |
| limonene | C10H16 | 176 | Solvent; fragrance; cleaning product ingredient | Limonene (C10H16) is a bicyclic terpene alkene common in citrus peel oils, used as a solvent and fragrance. It’s a clear liquid obtained from citrus waste, notable for one or more non-aromatic double bonds and a pleasant citrus scent. |
| alpha-pinene | C10H16 | 156 | Fragrance; terpene feedstock; industrial chemical | alpha-Pinene (C10H16) is a bicyclic monoterpene alkene abundant in pine resin and essential oils. Used as a fragrance and chemical feedstock, it’s notable for a strained double bond in a ring system that reacts easily in terpene chemistry. |
Images and Descriptions

ethene
Ethene is a simple two-carbon alkene (C2H4), a colorless gas used to make plastics and ripen fruit commercially. Produced by cracking hydrocarbons, it’s notable as the simplest alkene and contains one carbon–carbon double bond that readily polymerizes.

propene
Propene is a three-carbon alkene (C3H6), a flammable gas used to make polypropylene and other chemicals. Manufactured by steam cracking or as a refinery product, it’s notable for its double bond that undergoes polymerization and many addition reactions.

1-butene
1-Butene (C4H8) is a four-carbon alpha-olefin used as a comonomer in polyethylene production and for synthesis. It is a colorless gas or volatile liquid, notable for its terminal double bond that reacts in polymerization and hydroformylation.

cis-2-butene
cis-2-Butene (C4H8) is a branched four-carbon alkene present as a gas or liquid and used as petrochemical feedstock. It differs stereochemically from the trans isomer, with methyl groups on the same side, affecting boiling point and reactivity.

trans-2-butene
trans-2-Butene (C4H8) is a geometric isomer of 2-butene used in organic synthesis and petrochemicals. The methyl groups are on opposite sides of the double bond, giving a slightly lower boiling point and distinct physical properties from the cis isomer.
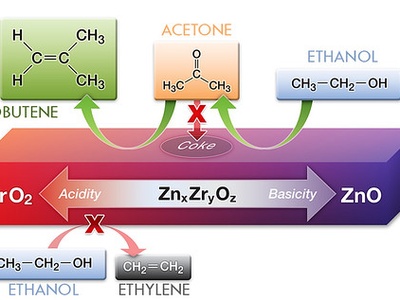
isobutene
Isobutene (2-methylpropene, C4H8) is a gaseous branched alkene used to make butyl rubber and methyl tert-butyl ether. Produced in refineries, it’s notable for a substituted carbon bearing the double bond, making it reactive in polymerizations.

1-pentene
1-Pentene (C5H10) is a terminal five-carbon alkene used as an intermediate in making plastics and fine chemicals. It is a volatile liquid produced by cracking and oligomerization, and reacts readily at its double bond in addition reactions.
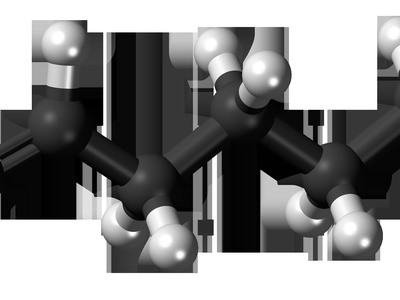
1-hexene
1-Hexene (C6H12) is a common alpha-olefin used as comonomer in polyethylene and in surfactant synthesis. It is a colorless liquid made industrially by oligomerizing ethylene or separating from C6 fractions in refineries.

1-octene
1-Octene (C8H16) is a higher alpha-olefin used to produce polyethylene comonomers and plasticizers. It is a clear liquid obtained by oligomerization or from wax cracking, notable for its terminal double bond allowing selective chemical modification.

1-decene
1-Decene (C10H20) is a long-chain alpha-olefin used in detergents, lubricants, and polymer comonomers. Produced industrially by oligomerizing ethylene, it’s a liquid notable for its reactive terminal double bond used in hydroformylation and alkylation chemistry.

1,3-butadiene
1,3-Butadiene (C4H6) is a conjugated diene used to make synthetic rubbers and plastics. It is a gas at room temperature, produced by cracking, notable for two double bonds that enable polymerization and Diels–Alder reactions.

isoprene
Isoprene (2-methyl-1,3-butadiene, C5H8) is a natural diene used to make natural and synthetic rubber. Emitted by plants and produced industrially, it’s notable for two conjugated double bonds that make it highly reactive in polymer chemistry.

styrene
Styrene (C8H8) is a vinyl-substituted aromatic monomer used to make polystyrene plastics and resins. Produced from ethylbenzene, it’s a liquid notable for a vinyl (C=C) group attached to a benzene ring that readily polymerizes.

cyclohexene
Cyclohexene (C6H10) is a six-membered cyclic alkene used as a solvent and chemical intermediate. Found in petroleum and made by hydrogenation routes, it’s notable for one non-aromatic double bond in a ring that undergoes addition reactions.

cyclopentene
Cyclopentene (C5H8) is a five-membered cyclic alkene used in organic synthesis and material precursors. It is a volatile liquid produced from petroleum fractions, notable for its ring double bond which is reactive in hydrogenation and polymer chemistry.

limonene
Limonene (C10H16) is a bicyclic terpene alkene common in citrus peel oils, used as a solvent and fragrance. It’s a clear liquid obtained from citrus waste, notable for one or more non-aromatic double bonds and a pleasant citrus scent.

alpha-pinene
alpha-Pinene (C10H16) is a bicyclic monoterpene alkene abundant in pine resin and essential oils. Used as a fragrance and chemical feedstock, it’s notable for a strained double bond in a ring system that reacts easily in terpene chemistry.