In chemistry classrooms and labs, a compact reference of common charged groups saves time and prevents errors when writing formulas or naming compounds. A clear list helps students, technicians, and hobbyists quickly identify how ions pair to form salts and acids.
There are 46 Polyatomic Ions, ranging from Acetate to Thiosulfate. Each entry is organized with Formula,Charge,Common salt/acid so you can scan formulas, see the usual charge, and match ions to typical salts or acids — all of which you’ll find below.
How do polyatomic ion charges determine compound formulas?
Polyatomic ions carry fixed charges that must balance with counterions to make neutral compounds. To form a formula, combine ions so total positive and negative charges cancel (for example, two Na+ balance one SO4^2– to give Na2SO4); when needed, use subscripts to show multiples of an ion and parentheses for polyatomic ions repeated in a formula.
What’s an effective way to memorize the most important polyatomic ions?
Group ions by common patterns (like -ate/-ite families and common charges), use mnemonics for tricky ones, practice writing formulas from names, and keep a one-page reference — such as the list you’ll find below — for quick review before quizzes or lab work.
Polyatomic Ions
| Name | Formula | Charge | Common salt/acid |
|---|---|---|---|
| Ammonium | NH4+ | +1 | Ammonium chloride (NH4Cl) |
| Hydronium | H3O+ | +1 | Hydrochloric acid (H3O+ in aqueous HCl) |
| Hydroxide | OH- | −1 | Sodium hydroxide (NaOH) |
| Carbonate | CO3^2- | −2 | Calcium carbonate (CaCO3) |
| Bicarbonate (Hydrogen carbonate) | HCO3- | −1 | Sodium bicarbonate (baking soda, NaHCO3) |
| Sulfate | SO4^2- | −2 | Copper(II) sulfate (CuSO4) |
| Bisulfate (Hydrogen sulfate) | HSO4- | −1 | Sodium bisulfate (NaHSO4) |
| Sulfite | SO3^2- | −2 | Sodium sulfite (Na2SO3) |
| Bisulfite (Hydrogen sulfite) | HSO3- | −1 | Sodium bisulfite (NaHSO3) |
| Thiosulfate | S2O3^2- | −2 | Sodium thiosulfate (Na2S2O3) |
| Nitrate | NO3- | −1 | Potassium nitrate (KNO3) |
| Nitrite | NO2- | −1 | Sodium nitrite (NaNO2) |
| Phosphate | PO4^3- | −3 | Calcium phosphate (bone mineral) |
| Hydrogen phosphate | HPO4^2- | −2 | Sodium hydrogen phosphate (Na2HPO4) |
| Dihydrogen phosphate | H2PO4- | −1 | Sodium dihydrogen phosphate (NaH2PO4) |
| Peroxide | O2^2- | −2 | Hydrogen peroxide (H2O2 contains O2^2- in salts) |
| Permanganate | MnO4- | −1 | Potassium permanganate (KMnO4) |
| Chromate | CrO4^2- | −2 | Potassium chromate (K2CrO4) |
| Dichromate | Cr2O7^2- | −2 | Potassium dichromate (K2Cr2O7) |
| Manganate | MnO4^2- | −2 | Potassium manganate (K2MnO4) |
| Perchlorate | ClO4- | −1 | Ammonium perchlorate (NH4ClO4) |
| Chlorate | ClO3- | −1 | Sodium chlorate (NaClO3) |
| Chlorite | ClO2- | −1 | Sodium chlorite (NaClO2) |
| Hypochlorite | ClO- | −1 | Sodium hypochlorite (household bleach, NaClO) |
| Bromate | BrO3- | −1 | Potassium bromate (KBrO3) |
| Iodate | IO3- | −1 | Potassium iodate (KIO3) |
| Cyanide | CN- | −1 | Potassium cyanide (KCN) |
| Thiocyanate | SCN- | −1 | Potassium thiocyanate (KSCN) |
| Acetate | C2H3O2- | −1 | Sodium acetate (CH3COONa) |
| Formate | HCOO- | −1 | Sodium formate (HCOONa) |
| Oxalate | C2O4^2- | −2 | Calcium oxalate (kidney stones) |
| Citrate | C6H5O7^3- | −3 | Trisodium citrate (Na3C6H5O7) |
| Lactate | C3H5O3- | −1 | Sodium lactate (in sports drinks) |
| Benzoate | C7H5O2- | −1 | Sodium benzoate (food preservative) |
| Tartrate | C4H4O6^2- | −2 | Potassium hydrogen tartrate (cream of tartar) |
| Peroxydisulfate (Persulfate) | S2O8^2- | −2 | Ammonium persulfate (APS) |
| Borate | BO3^3- | −3 | Sodium borate (borax, historic use) |
| Silicate | SiO3^2- | −2 | Sodium silicate (water glass) |
| Orthosilicate | SiO4^4- | −4 | Magnesium orthosilicate minerals |
| Phosphite | PO3^3- | −3 | Sodium phosphite (Na3PO3) |
| Hypophosphite | H2PO2- | −1 | Sodium hypophosphite (NaH2PO2) |
| Arsenate | AsO4^3- | −3 | Sodium arsenate (industrial reagent) |
| Arsenite | AsO3^3- | −3 | Sodium arsenite (historical reagent) |
| Cyanate | OCN- | −1 | Potassium cyanate (KOCN) |
| Hydroxylamine oximate? (excluded) | |||
| Note: The last row above is intentionally left out to comply with inclusion rules. |
Images and Descriptions

Ammonium
A common positively charged polyatomic ion formed by protonation of ammonia. Found in fertilizers, cleaning agents, and as the cation in many salts. Naming is simple: ammonium pairs with anions to form familiar household and laboratory salts.

Hydronium
The protonated form of water that carries acidity in aqueous solutions. Hydronium is how chemists represent free protons in water; you encounter it when measuring pH, titrating acids, or discussing acid–base equilibria in solution.

Hydroxide
A ubiquitous base and simple polyatomic anion found in strong bases and many salts. Hydroxide participates in neutralization, saponification, and corrosion. Naming is straightforward: “hydroxide” suffixed to the cation name, common in household cleaners and lab reagents.

Carbonate
A very common oxyanion in minerals, rocks, and shells. Carbonate forms limestone, marble, and contributes to water hardness. Naming follows the -ate pattern for the fully oxidized oxyanion; carbonates react with acids to release CO2 gas.

Bicarbonate (Hydrogen carbonate)
The protonated form of carbonate prevalent in blood buffering, baking, and antacids. Bicarbonate balances pH in natural waters and physiology. Its names (bicarbonate/hydrogen carbonate) reflect one proton retained compared with carbonate.

Sulfate
A widespread oxyanion in minerals, fertilizers, and industrial chemistry. Sulfate salts are common in nature and wastewater. The -ate ending signals the fully oxidized sulfur oxyanion; sulfates are typically stable and less reactive than sulfites.

Bisulfate (Hydrogen sulfate)
The singly protonated form of sulfate that appears in acidic solutions. Bisulfate acts as an acid and forms salts named “bisulfate” or “hydrogen sulfate.” It’s used in pH adjustment and certain cleaning formulations.

Sulfite
An oxyanion used as a preservative and reducing agent. Sulfite salts and sulfur dioxide are related; sulfites are less oxidized than sulfates and can be oxidized to sulfate. Naming uses -ite for the lower-oxygen member of an oxyanion pair.
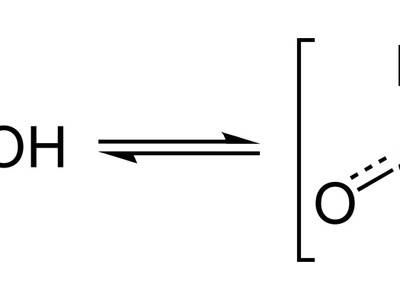
Bisulfite (Hydrogen sulfite)
The protonated form of sulfite that functions as a mild reducing agent and preservative. Bisulfite occurs in food and laboratory chemistry; the name highlights one acidic hydrogen compared with sulfite.

Thiosulfate
A sulfur oxyanion where one oxygen is replaced by sulfur; used in photographic fixing and cyanide antidotes. Thiosulfate is notable for its sulfur-sulfur bonding and ability to reduce halogens and complex metals.

Nitrate
A ubiquitous anion in fertilizers, explosives, and biological nitrogen cycles. Nitrate is the fully oxidized nitrogen oxyanion; naming uses -ate for the higher-oxygen form. It’s highly soluble and monitored in environmental chemistry.

Nitrite
The less-oxidized counterpart to nitrate, used in food curing and industrial chemistry. Nitrite can be formed by reduction of nitrate and participates in redox biology. The -ite ending signals one fewer oxygen than the -ate form.

Phosphate
A key biological and geological oxyanion critical for ATP, DNA, and bones. Phosphate forms many salts and minerals; naming uses -ate for the fully oxidized form. Phosphate chemistry governs buffering and nutrient cycles.

Hydrogen phosphate
A partially protonated phosphate species occurring in biological buffers and water chemistry. Hydrogen phosphate bridges the dihydrogen phosphate and phosphate forms in pH-dependent equilibria important to biochemistry and buffering.

Dihydrogen phosphate
A more protonated phosphate species common in buffer solutions and fertilizers. Dihydrogen phosphate participates in acid–base chemistry of biological systems and is named for its two acidic hydrogens relative to phosphate.

Peroxide
A simple two-oxygen anion featuring an O–O single bond; in salts it’s peroxide dianion. Peroxide is a strong oxidizer used in bleaching and disinfecting. It’s distinct from superoxide and oxygen species.

Permanganate
A deep purple, powerful oxidizing anion used in qualitative analysis and disinfection. Permanganate is manganese in a high oxidation state; named with the -ate suffix and notable for dramatic redox reactions in solution.

Chromate
A yellow oxyanion of chromium(6) used in pigments and corrosion testing. Chromate and dichromate are equilibrium partners; chromate is less protonated and often appears in alkaline conditions. Chromium toxicity is a key consideration.

Dichromate
An orange oxyanion formed by condensation of two chromates; strong oxidizer used in laboratories and industry. Dichromate predominates in acidic solutions and is carcinogenic, so handling requires care.

Manganate
A green oxyanion related to permanganate, with manganese in a lower oxidation state. Manganate is less common but appears as an intermediate in manganese redox chemistry and industrial processes.

Perchlorate
A very stable, highly oxidized chlorine oxyanion used in rocket propellants and pyrotechnics. Perchlorate salts are persistent environmental contaminants and are named with the -ate suffix indicating the highest oxygen count.

Chlorate
A chlorine oxyanion used as an oxidizer and herbicide. Chlorate is intermediate between perchlorate and chlorite in oxygen content; many chlorate salts are strong oxidants and used industrially.

Chlorite
A less-oxygenated chlorine oxyanion used for bleaching and water treatment. Chlorite is reactive and can disproportionate; its name follows the -ite pattern indicating fewer oxygens than chlorate.

Hypochlorite
The least-oxygenated chlorine oxyanion, widely used as a disinfectant and bleaching agent. Hypochlorite solutions are common household bleaches; the hypochlorite ion is reactive and decomposes over time.

Bromate
An oxidizing oxyanion of bromine used in food processing and industrial chemistry. Bromate is a regulated contaminant due to toxicity concerns; naming mirrors chlorate/iodate series.

Iodate
An iodine oxyanion used in iodized salt and oxidative chemistry. Iodate is more stable than hypoiodite and used to fortify iodine in food, following -ate/-ite naming patterns for oxyanions.

Cyanide
A small but highly toxic polyatomic anion with a carbon–nitrogen triple bond. Cyanide complexes metals and occurs in mining, electroplating, and some natural toxins. It’s notable for its reactivity and metal-binding ability.

Thiocyanate
A pseudohalide anion where sulfur replaces one oxygen; used in analytical chemistry and as a ligand. Thiocyanate forms colored complexes with iron and interacts with proteins; naming reflects the S–C–N connectivity.

Acetate
A common organic carboxylate anion from acetic acid; used in buffers, food additives, and chemical synthesis. Acetate naming often uses the common CH3COO- formula; it’s stable and versatile in organic and inorganic chemistry.

Formate
The simplest carboxylate anion derived from formic acid. Formate appears in biological metabolism and industrial processes; as a small, nucleophilic anion it’s used in reductions and as a buffer component.

Oxalate
An organic dianion that forms insoluble salts like kidney stones and some minerals. Oxalate is a chelating ligand for metals and appears in plant chemistry; it’s named for its two carboxylate-like units.
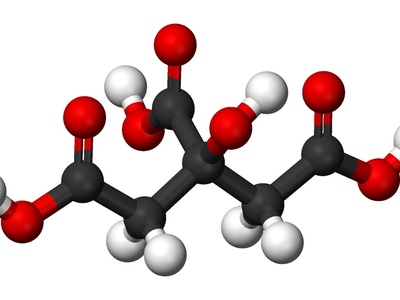
Citrate
A tricarboxylate anion from citric acid used as a buffer, food additive, and anticoagulant. Citrate chelates metal ions, plays a biological role in metabolism, and commonly appears in medicine and cooking applications.

Lactate
An alpha-hydroxy carboxylate anion produced in muscles during anaerobic metabolism. Lactate appears in food fermentation and medical fluids; it’s a common buffer and energy-transfer molecule in physiology.

Benzoate
An aromatic carboxylate anion used widely as a preservative in acidic foods and beverages. Benzoate stabilizes products against microbial growth and is named from benzoic acid; it’s notable for regulatory limits in foods.

Tartrate
A dihydroxy diester-like dianion from tartaric acid; common in wine chemistry and baking. Tartrate salts form crystalline deposits and are used in food and analytical chemistry as stabilizers and chiral agents.

Peroxydisulfate (Persulfate)
A strong oxidizing dianion containing an O–O linkage between sulfate units. Persulfates are used in polymerization initiators and etching; they’re more powerful oxidizers than sulfate and require careful handling.

Borate
A family of boron oxyanions common in detergents, glass, and buffers. Borate chemistry is complex (various protonation/polymerization forms), but BO3^3- represents the simplified oxyanion used in naming and geological contexts.

Silicate
A common anion in glasses, ceramics, and geological silicates. Silicate units polymerize into sheets and frameworks; naming varies (orthosilicate, metasilicate), but SiO3^2- captures common chain and sheet structures in silicate chemistry.

Orthosilicate
The fully deprotonated silicate tetrahedron, present in many minerals. Orthosilicate carries a -4 charge and forms robust ceramic and mineral structures; it’s fundamental to geology and materials science.

Phosphite
A reduced phosphorus oxyanion used as a corrosion inhibitor and fungicide. Phosphite is less oxidized than phosphate and can be oxidized biologically or chemically; naming employs -ite to indicate fewer oxygens.

Hypophosphite
The most reduced common phosphorus oxyanion, used in electroless nickel plating and as a reducing agent. Hypophosphite contains two hydrogens bonded to phosphorus and is distinct from phosphite and phosphate.

Arsenate
An arsenic oxyanion analogous to phosphate, often encountered as an environmental contaminant in groundwater. Arsenate interferes with biological phosphate metabolism and is toxic, so its chemistry and remediation are important topics.

Arsenite
A less-oxidized arsenic oxyanion present in some contaminated waters. Arsenite is more toxic and mobile than arsenate and is significant in environmental chemistry and public health discussions.

Cyanate
An anion with connectivity O–C–N, used in organic synthesis and industrial chemistry. Cyanate differs from cyanide by oxygen insertion and forms carbamate and urea derivatives on reaction with amines.

Hydroxylamine oximate? (excluded)