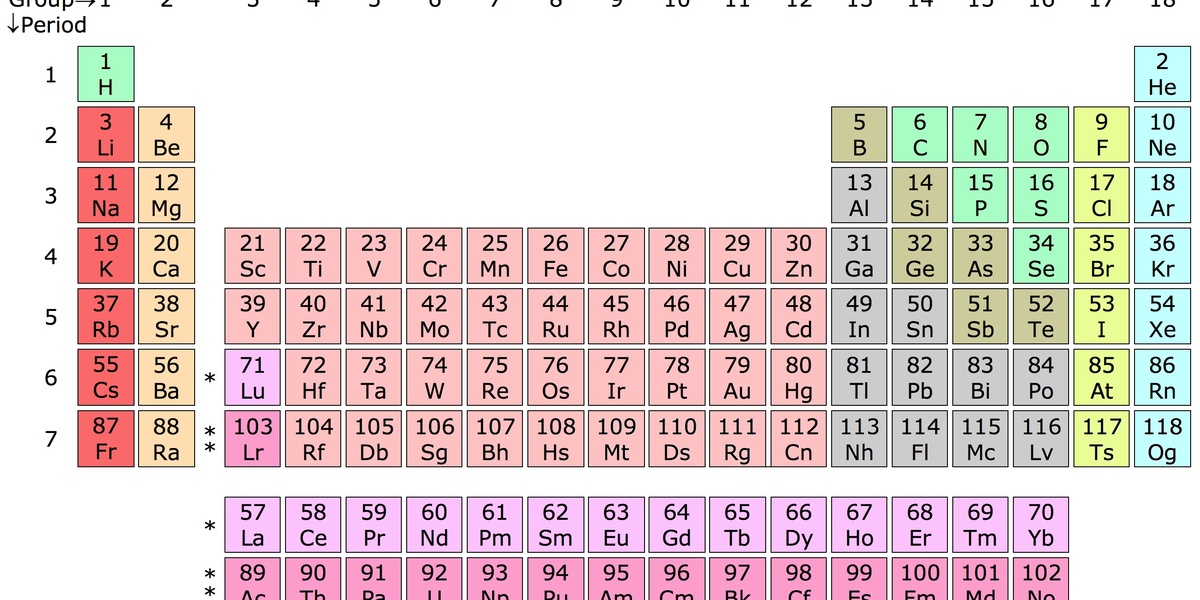
In 1981 researchers first produced element 107, a brief but pivotal moment that launched a handful of chemistry experiments probing how this synthetic metal behaves. Although atoms of element 107 live for only seconds to minutes under laboratory conditions, experiments and theory have identified a small set of elements bohrium reacts with in measurable ways that help chemists place it in the periodic table.
Why care? Because reactions that can be observed atom-by-atom test periodic trends, inform nuclear chemistry, and improve theoretical models used across heavy-element science. This piece examines eight specific partners, grouped into three categories: fundamental chemical partners (oxygen and halogens), experimental partners and surfaces (gold, platinum, iodine), and comparative or theoretical partners (sulfur, hydrogen, bromine).
Expect references to gas-phase chromatography, alpha-decay tagging at facilities like GSI and JINR, comparisons with technetium and rhenium, and a few quantitative anchors — for example, atomic number 107, the discovery year 1981, and the atom-by-atom timescales typical of these studies.
Fundamental Chemical Partners
Bohrium sits in group 7 beneath technetium and rhenium, so group trends guide expectations about how it will bond with nonmetals. Much of the observable chemistry for transactinides comes from creating volatile oxides or halides and following them through a gas stream, often capturing single atoms on detector surfaces.
Experiments at places such as GSI Darmstadt during the 1990s–2000s pioneered gas-phase chromatography methods for these short-lived isotopes, and comparisons to Re and Tc chemistry remain a baseline for interpretation.
1. Oxygen (O) — Oxidation states and oxy-compounds
Bohrium shows oxidized chemistry consistent with group 7 behavior, and oxygen interactions are central to the observed chemistry. Researchers look for volatile oxy-species because they resemble what rhenium and technetium do in analogous oxidation states.
Gas-phase chromatography experiments detect oxide-like volatility by moving atoms in an oxygen-containing carrier gas and recording where they adsorb; these single-atom experiments run on timescales of seconds and use alpha-decay tagging to identify capture events. Detecting an oxy-species helps place bohrium among its homologues and constrains ab initio models that predict stable oxides for transactinides.
2. Chlorine (Cl) — Formation of volatile chlorides
Chloride chemistry — especially volatile oxychlorides — provides some of the clearest experimental pathways for bohrium. In many experiments chlorine gas or HCl is mixed into a carrier stream to produce Bh–Cl or Bh–OCl species that can be separated by volatility.
GSI experiments from the 1990s–2000s and follow-on work used gas-phase chromatographic separation of volatile chlorides to capture single atoms on detector surfaces in under a minute. Because chlorination yields volatile species for Re and Tc, observing similar behavior for bohrium strengthens its placement in group 7.
3. Bromine (Br) — Halogen trends and heavier halide behavior
Bromine sits just below chlorine among the halogens and is useful for testing how bond strength and volatility evolve with halogen size. Theoretical bond-energy estimates for Bh–Br versus Bh–Cl help predict whether bromides will be sufficiently volatile to detect in gas-phase setups.
Bromides are often predicted to be less volatile than chlorides, but even failed or marginal bromination experiments are informative: they refine the potential-energy surfaces plugged into relativistic quantum-chemical models and provide comparative data against rhenium and technetium bromides.
Experimental Partners and Surfaces
Many reactions reported for bohrium are really interactions with experimental surfaces and detector materials rather than bulk chemistry in solution. Adsorption and deposition onto metals determine whether a single atom will stick long enough to be recorded.
Choice of detector material, temperature windows, and carrier-gas composition often dictate what chemists observe. That’s why surface chemistry — not just classic compound formation — plays an outsized role in transactinide studies.
4. Gold (Au) — Surface adsorption and detection
Gold-plated detectors are routinely used because noble-metal surfaces give reproducible adsorption behavior for single atoms. Bohrium atoms that form volatile species often end up captured on gold where their alpha decay is recorded to tag an event.
Adsorption-energy measurements and comparative studies (Bh vs. Re or Hs) let researchers infer relative bond strengths. Alpha-spectroscopy using gold-plated detectors yields single-atom adsorption events on millisecond-to-second timescales, enabling chemical identification despite extremely low production rates.
5. Platinum (Pt) — Catalytic surfaces and comparative adsorption
Platinum surfaces are sometimes used as an alternative to gold to probe surface bonding and to mimic catalytic environments. Comparative adsorption on Pt versus Au refines our view of bohrium’s surface chemistry and helps test theoretical adsorption models.
Some chromatography columns include platinum segments or wires, and theoretical work reports adsorption-energy ratios that guide which surface will most likely capture a Bh atom at a given temperature window. These choices directly affect experimental yields and the prospects for observing new reactions.
6. Iodine (I) — Heavy halide interactions and volatility contrasts
Iodine, the heaviest common halogen discussed here, is a useful probe of how Bh–halogen bonding weakens or strengthens with halogen size. Theoretical Bh–I bond-energy estimates indicate iodides may be the least volatile of the common Bh halides, but that information still constrains models.
Contrasting iodine with chlorine and bromine across three halogens in gas-phase or halogen-exchange studies narrows the possible bonding scenarios and helps experimenters pick conditions likely to yield observable adsorption or capture events.
Comparative and Theoretical Partners
Where experiments struggle — because isotopes are short-lived and made atom-by-atom — theory and comparison to lighter homologues fill in the gaps. Ab initio, relativistic quantum-chemical calculations predict which ligands might bind and what oxidation states are accessible.
Comparing Bh to technetium (element 43) and rhenium (element 75) remains central; those comparisons explain experimental choices at facilities such as GSI Darmstadt and JINR Dubna and help prioritize future tests.
7. Sulfur (S) — Predicted sulfide behavior and ligand chemistry
Sulfur ligands and sulfides are predicted to bind bohrium in oxidation states analogous to rhenium sulfides, but direct experimental evidence is scarce. The main obstacles are short half-lives and low production rates that make solution chemistry nearly impossible for many isotopes.
Ab initio predictions for Bh–S bond strengths, informed by Re chemistry, help researchers decide whether to attempt ligand-exchange or complexation experiments. Remember: Bh is element 107 while Re is 75, and those 32 atomic-number differences lead to significant relativistic effects that theory must account for.
8. Hydrogen (H) — Hydride predictions and gas-phase interactions
Hydride formation for bohrium is mostly a theoretical possibility, explored in computational studies that model Bh–H bonding and gas-phase collisions. If hydrides formed and were volatile, they’d change predictions for capture probabilities and detection strategies.
Comparisons to rhenium and technetium hydrides give a starting point, but any experimental test would need optimized production, sub-second transport, and carefully chosen surfaces to trap and tag single atoms. Overall, hydrogen interactions remain an area where theory points experiments forward.
Summary
- Bohrium’s observable chemistry comes from both direct single-atom experiments and detailed relativistic theory; only a handful of partners (8 discussed here) yield measurable signals.
- Oxygen and halogens (Cl, Br, I) produce the clearest volatile species, and chloride/oxychloride routes have been especially productive in gas-phase chromatography at facilities like GSI and JINR.
- Gold and platinum surfaces act as functional “reactive partners” in practice — adsorption on Au often determines whether an atom is captured and identified.
- Comparisons with technetium and rhenium and targeted ab initio work (sulfur and hydride predictions, for example) guide future experiments and help interpret atom-by-atom results obtained on seconds-to-minutes timescales.