If you’ve ever worked in a teaching lab, stocked a research bench, or mixed household solutions, a tidy list of common chemicals saves time and reduces guesswork. Having quick access to names, formulas and solubility helps when deciding what will dissolve, how to prepare solutions, and what safety steps to take.
There are 32 Solutes, ranging from Acetic acid to Urea. Each entry is organized with the columns Formula,Category,Solubility in water (g/100 mL at 20 C), so you can quickly match a compound to its chemical formula, type, and how much will dissolve at room temperature — you’ll find below.
What does “solubility in water (g/100 mL at 20 C)” tell me and how precise is it?
That value means grams of solute that dissolve in 100 mL of water at 20 °C; treat it as a practical reference rather than an absolute limit, since solubility changes with temperature, purity and experimental conditions — use it to estimate whether a compound will dissolve easily or require heating/solvent adjustments.
How can I use the Formula and Category columns when preparing solutions?
Use the Formula to confirm you have the correct chemical and the Category to understand chemical family, typical behavior and safety considerations; combine that with the solubility number to pick appropriate concentrations, solvents, and handling measures before preparing solutions.
Solutes
| Name | Formula | Category | Solubility in water (g/100 mL at 20 C) |
|---|---|---|---|
| Sodium chloride | NaCl | ionic salt | 35.90 |
| Potassium chloride | KCl | ionic salt | 34.00 |
| Calcium chloride | CaCl2 | ionic salt | 74.50 |
| Magnesium sulfate | MgSO4 | ionic salt | 25.00 |
| Ammonium nitrate | NH4NO3 | ionic salt | 121.00 |
| Sodium bicarbonate | NaHCO3 | ionic salt | 9.60 |
| Sodium carbonate | Na2CO3 | ionic salt | 21.50 |
| Potassium nitrate | KNO3 | ionic salt | 13.30 |
| Copper(II) sulfate | CuSO4 | ionic salt | 24.00 |
| Sodium hydroxide | NaOH | ionic salt | 111.00 |
| Hydrogen chloride | HCl | gas | miscible |
| Carbon dioxide | CO2 | gas | sparingly soluble |
| Oxygen | O2 | gas | sparingly soluble |
| Ammonia | NH3 | gas | moderately soluble |
| Ethanol | C2H5OH | small organic | miscible |
| Methanol | CH3OH | small organic | miscible |
| Acetone | C3H6O | small organic | miscible |
| Acetic acid | CH3COOH | small organic | miscible |
| Urea | CH4N2O | small organic | 107.00 |
| Glycerol | C3H8O3 | small organic | miscible |
| Ethylene glycol | C2H6O2 | small organic | miscible |
| Benzene | C6H6 | small organic | 0.18 |
| Phenol | C6H5OH | small organic | 8.30 |
| Glucose | C6H12O6 | biomolecule | 91.00 |
| Sucrose | C12H22O11 | biomolecule | 200.00 |
| Glycine | NH2CH2COOH | biomolecule | 24.90 |
| Adenosine triphosphate | C10H16N5O13P3 | biomolecule | soluble |
| Fructose | C6H12O6 | biomolecule | 79.00 |
| Sodium ion | Na+ | metal ion | soluble (aqueous ion) |
| Calcium ion | Ca2+ | metal ion | soluble (aqueous ion) |
| Iron(III) ion | Fe3+ | metal ion | sparingly soluble (depends on anion/pH) |
| Copper(II) ion | Cu2+ | metal ion | soluble (as salts) |
Images and Descriptions

Sodium chloride
Common table salt; simple ionic compound widely used for seasoning, food preservation, and deicing. Dissolves readily in water to give Na+ and Cl− ions and is a key electrolyte in biology and many industrial processes.

Potassium chloride
A common source of potassium for fertilizers and medical electrolytes; dissolves in water to give K+ and Cl−. Used in agriculture, food processing, and as a low-sodium salt substitute in cooking.

Calcium chloride
Highly water-soluble de-icing agent and desiccant used in road treatment, dust control, and concrete acceleration. Dissolves to yield Ca2+ and Cl− ions and is used in food as a firming agent and electrolyte source.

Magnesium sulfate
Known as Epsom salt in its hydrated form; used in bath salts, laxatives, agriculture, and as a laboratory reagent. Dissolves to give Mg2+ and SO42− and is used medically for magnesium supplementation.

Ammonium nitrate
A highly soluble inorganic fertilizer and oxidizer used in agriculture and industrial explosives (with restrictions). Dissolves readily to give NH4+ and NO3− and is notable for its hygroscopic nature and nitrate supply.

Sodium bicarbonate
Baking soda; used in cooking, cleaning, antacids, and laboratory buffers. Moderately soluble in water, it releases bicarbonate ions and is used to neutralize acids or as a mild leavening agent.

Sodium carbonate
Known as soda ash; used in glassmaking, water softening, and cleaning products. Dissolves in water to give carbonate ions, raises pH, and is an important industrial alkali.

Potassium nitrate
A fertilizer and oxidizing agent historically used in gunpowder; dissolves in water to supply K+ and NO3−. Used in food curing, pyrotechnics, and some laboratory applications.

Copper(II) sulfate
Blue crystalline salt used as a fungicide, algicide, and laboratory reagent. Dissolves to give Cu2+ and SO42− ions; notable for its color and use in education for crystal demonstrations and copper chemistry.

Sodium hydroxide
Strong inorganic base used in soapmaking, chemical manufacturing, drain cleaners, and pH control. Highly soluble in water, producing strongly alkaline solutions and releasing heat on dissolution.

Hydrogen chloride
Hydrogen chloride is a gas that dissolves readily in water to form hydrochloric acid; widely used in chemical synthesis, pH adjustment, industrial pickling and household cleaners. In solution it supplies Cl− and strong acidity.
Carbon dioxide
A common dissolved gas in natural waters and beverages; reacts partially to form carbonic acid and bicarbonate. Moderately soluble relative to other gases and central to respiration, carbonation, and global carbon cycling.
Oxygen
Essential dissolved gas for aquatic life, oxygen has low solubility in water that depends on temperature and pressure. Supplied to rivers and aquaria by aeration; critical to respiration and many oxidation reactions in water.

Ammonia
Ammonia gas dissolves readily in water to give a basic solution (NH3/NH4+ equilibrium). Used in fertilizers, refrigeration, and cleaners; soluble ammonia is important in environmental nitrogen cycling and industrial chemistry.

Ethanol
Common alcohol in beverages, solvents, and fuels; completely miscible with water. Widely used as an antiseptic, solvent, and intermediary in chemical synthesis; notable for pleasant taste when diluted and volatility when concentrated.

Methanol
Simplest alcohol used as an industrial solvent, fuel, and chemical feedstock. Fully miscible with water and toxic to humans if ingested. Important in biodiesel production and as a laboratory reagent.

Acetone
A volatile, water-miscible solvent used in nail polish removers, cleaning, and organic synthesis. Readily dissolves many organic compounds and is a common laboratory solvent thanks to its high volatility and solvent power.

Acetic acid
The main component of vinegar, acetic acid is water-miscible and used in food, chemical synthesis, and as a pH adjusting agent. Weak acid behavior and smell make it familiar in kitchens and laboratories alike.

Urea
A highly water-soluble small organic used as fertilizer, in wastewater treatment, and as a protein denaturant in labs. Readily dissolves to give neutral, hydrogen-bonding molecules and is a major nitrogen carrier in urine.

Glycerol
Viscous, sweet-tasting, water-miscible triol used in cosmetics, food humectants, and pharmaceuticals. Highly soluble and hygroscopic, glycerol stabilizes formulations and serves as a benign solvent and cryoprotectant in laboratories.
Ethylene glycol
Common antifreeze and industrial solvent, ethylene glycol is fully miscible with water and depresses freezing point. Toxic to humans and pets if ingested, it is widely used in cooling systems and chemical processes.

Benzene
A nonpolar aromatic hydrocarbon that is sparingly soluble in water; widely used as an industrial precursor and solvent but highly toxic and carcinogenic. Often avoided in consumer products and handled with strict safety controls.

Phenol
An aromatic compound used in resins, disinfectants, and chemical synthesis. Moderately soluble in water and acidic; phenol is caustic and must be handled carefully. Historically important in antiseptic development and industrial chemistry.
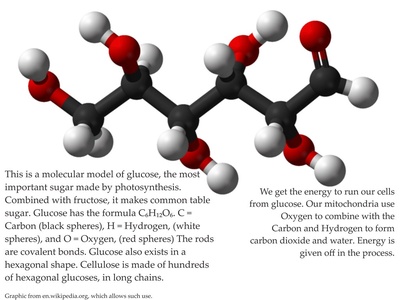
Glucose
A central monosaccharide and primary biological energy source; highly soluble in water and ubiquitous in foods, metabolism, and fermentation. Used in medicine, food science, and labs as a standard carbohydrate and energy substrate.

Sucrose
Table sugar, a disaccharide commonly dissolved in beverages, syrups, and cooking. Very soluble in water and widely used as a sweetener and preservative; important in food chemistry and fermentation processes.

Glycine
The simplest amino acid, glycine is water-soluble and used in buffer formulations, biological studies, and as a supplement. Neutral at physiological pH for some applications and a building block for proteins and metabolites.

Adenosine triphosphate
ATP is the cellular “energy currency,” soluble in water as charged phosphate salts. Central to metabolism, muscle contraction, and signaling; commonly handled in labs as sodium or magnesium salts for biochemical assays.

Fructose
A monosaccharide found in fruit and honey, fructose is highly soluble in water and used as a sweetener and metabolic fuel. Important in food science, beverage formulation, and studies of carbohydrate metabolism.
Sodium ion
A ubiquitous dissolved cation in natural waters and biological fluids, sodium ion arises from many soluble salts. Crucial for nerve function, osmotic balance, and industrial processes that rely on ionic conductivity.

Calcium ion
A doubly charged cation present in hard water and biological systems; it participates in bone formation, signaling, and water hardness. Dissolves as hydrated Ca2+ from soluble salts and is important in chemistry and physiology.

Iron(III) ion
Ferric ion forms colored aqueous species and precipitates depending on pH; important in corrosion, water treatment, and nutrition. Solubility is strongly pH- and ligand-dependent, often forming complexes in solution.

Copper(II) ion
A common transition-metal cation giving characteristic blue/green solutions; used in electroplating, catalysis, and biological studies. Solubility depends on counterions and pH but many copper salts dissolve readily in water.