In labs, fabs and research groups focused on electronics and materials, indium shows up in everything from infrared detectors to thin-film transistors. Its compounds bridge inorganic semiconductors and organometallic precursors, so a quick reference can save time when you need a specific formula or application.
There are 27 Indium Compounds, ranging from Indium antimonide to Trimethylindium. For each entry you’ll find below data organized as Formula,Oxidation state,Uses so you can quickly compare chemical identity, common oxidation states and typical applications — you’ll find below.
What are the most common applications of indium compounds?
Indium compounds are widely used in semiconductors (e.g., indium antimonide and indium phosphide for IR detectors and high-speed electronics), transparent conductive oxides (indium tin oxide in displays and touchscreens), and as precursors in metalorganic chemical vapor deposition (e.g., Trimethylindium) for thin films and LEDs.
How should I handle and store indium compounds safely?
Treat indium compounds according to their hazard data: use appropriate PPE, work in a fume hood for powders and volatile precursors, store reactive or pyrophoric materials under inert atmosphere as recommended, and consult the MSDS for disposal and emergency procedures.
Indium Compounds
| Name | Formula | Oxidation state | Uses |
|---|---|---|---|
| Indium(III) oxide | In2O3 | +3 | Transparent conductive oxides, catalysts |
| Indium tin oxide (ITO) | In2O3:SnO2 | +3 | Transparent electrodes for displays and photovoltaics |
| Indium(III) chloride | InCl3 | +3 | Synthesis precursor, catalysts, etchant |
| Indium(III) bromide | InBr3 | +3 | Precursor in synthesis, catalysts |
| Indium(III) iodide | InI3 | +3 | Organic synthesis, reagent |
| Indium(III) fluoride | InF3 | +3 | Optical ceramics, fluoride chemistry |
| Indium(III) hydroxide | In(OH)3 | +3 | Precursor to oxides, coatings |
| Indium(III) nitrate | In(NO3)3 | +3 | Precursor for thin films and catalysts |
| Indium(III) sulfate | In2(SO4)3 | +3 | Electroplating baths, pigment synthesis |
| Indium(III) acetate | In(CH3COO)3 | +3 | Precursor for sol–gel and inks |
| Trimethylindium | In(CH3)3 | +3 | MOCVD precursor for III–V semiconductors |
| Indium phosphide | InP | +3 | Lasers, LEDs, high-speed electronics |
| Indium arsenide | InAs | +3 | Infrared detectors, high-electron-mobility devices |
| Indium antimonide | InSb | +3 | Infrared detectors, Hall sensors, thermistors |
| Indium nitride | InN | +3 | High-electron-mobility devices, LEDs research |
| Indium(III) sulfide | In2S3 | +3 | Photovoltaic absorbers, pigments |
| Indium(III) selenide | In2Se3 | +3 | Phase-change memory, photovoltaics research |
| Indium(III) telluride | In2Te3 | +3 | Thermoelectrics research, semiconductors |
| Indium(I) iodide | InI | +1 | Specialty synthesis, precursor in metallurgy |
| Indium(I) chloride | InCl | +1 | Gas-phase chemistry, specialized synthesis |
| Indium monoselenide | InSe | +1,+3 | Layered semiconductor, photodetectors research |
| Indium monosulfide | InS | +1,+3 | Photovoltaics research, semiconductors |
| Indium telluride | InTe | +1,+2 | Semiconductor research, thermoelectrics |
| Indium oxyhydroxide | InO(OH) | +3 | Precursor to catalysts and oxides |
| Indium triflate | In(CF3SO3)3 | +3 | Lewis acid catalyst, organic synthesis |
| Indium perchlorate | In(ClO4)3 | +3 | Oxidizing agent, research reagent |
| Indium oxalate | In2(C2O4)3 | +3 | Precursor for materials synthesis |
Images and Descriptions

Indium(III) oxide
A wide-bandgap oxide used as a transparent conductor and ceramic material. It forms bixbyite and other polymorphs, is a major component of ITO, can be toxic in dust form, and is processed for thin films and sensors.

Indium tin oxide (ITO)
A conductive, transparent ceramic commonly used as a thin-film electrode on touchscreens, LCDs, and solar cells. It is a doped indium oxide solid solution, brittle, expensive due to indium scarcity, and recyclable but toxic in fine particulate form.
Indium(III) chloride
A hygroscopic white solid used as a Lewis acid, precursor to organoindium reagents, and in etching or electroplating. Dissolves in water to give acidic solutions; corrosive and harmful if inhaled or ingested; handled under inert atmosphere for organometallic chemistry.
Indium(III) bromide
A hygroscopic salt used as a brominating reagent and precursor to other indium compounds. It acts as a Lewis acid in organic synthesis; corrosive and moisture sensitive, typically handled under dry, inert conditions in laboratories and industry.
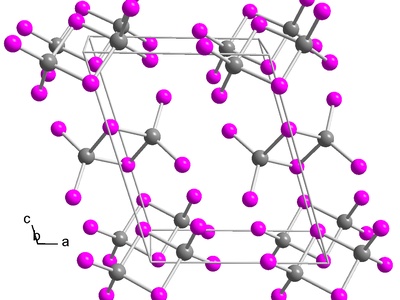
Indium(III) iodide
A red-orange to yellow solid applied as a halide source in synthesis and organoindium preparation. It is sensitive to moisture, moderately corrosive, and used under inert atmosphere for preparing catalysts and semiconductor precursors.

Indium(III) fluoride
A stable, high-melting inorganic fluoride used in specialty glasses and ceramics and as a fluorinating agent. Less soluble than other indium halides; corrosive and can release toxic fumes with strong acids or when heated.
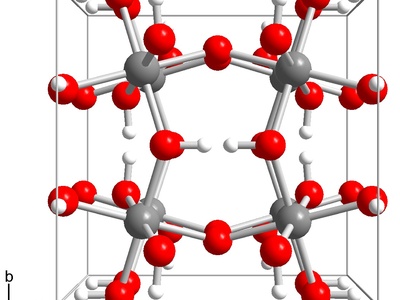
Indium(III) hydroxide
An amphoteric, gelatinous white solid that yields indium oxide on heating. Used as a precursor in sol–gel and precipitation routes for ceramics and thin films; low solubility and can cause irritation if inhaled as dust.
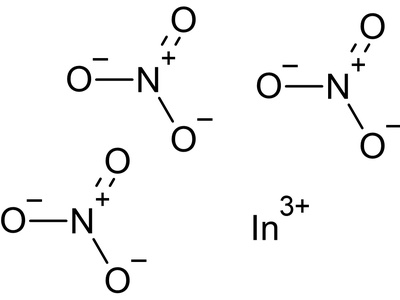
Indium(III) nitrate
A water-soluble salt employed as a precursor for sol–gel processes, thin-film deposition, and catalyst preparation. Decomposes on heating to indium oxide; corrosive and oxidizing, requiring careful handling to avoid skin and respiratory exposure.

Indium(III) sulfate
A soluble indium salt used in electroplating, pigment and catalyst manufacture, and laboratory chemistry. Solutions are acidic; salts are corrosive, and disposal must follow regulations to prevent environmental contamination with indium compounds.

Indium(III) acetate
An organometallic salt used as a precursor for sol–gel chemistry, nanoparticle synthesis, and printable conductive inks. Soluble in organic solvents; hygroscopic and moderately toxic, often decomposed to In2O3 for electronic applications.
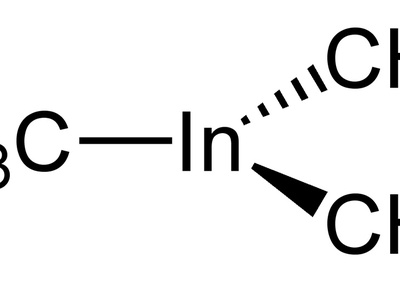
Trimethylindium
A volatile organometallic liquid used widely as a metalorganic chemical vapor deposition precursor for III–V semiconductor growth (e.g., InP). Pyrophoric and toxic; handled in gas/vapor lines with strict safety controls for deposition processes.

Indium phosphide
A III–V semiconductor with direct bandgap used in high-speed electronics, photonic devices, and telecom lasers. Grown by MOCVD or HVPE; requires toxic precursors and careful processing. Compounds can release phosphine and other hazardous gases during fabrication.

Indium arsenide
A narrow-bandgap III–V semiconductor prized for high electron mobility and infrared detection. Used in detectors, lasers, and research. Toxic arsenic-containing compounds and surface oxides require controlled handling and disposal in manufacturing environments.
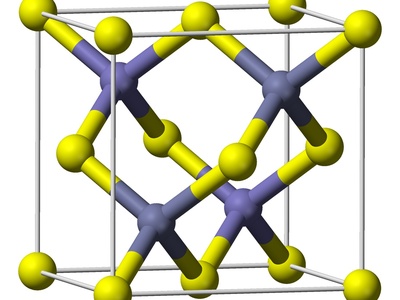
Indium antimonide
A very narrow-bandgap III–V semiconductor used for infrared detectors, Hall effect sensors, and low-temperature electronics. High electron mobility enables sensitive devices; antimony and indium compounds require careful waste control due to toxicity and environmental concerns.
Indium nitride
A III–V semiconductor with high electron mobility and potential for green–blue optoelectronics when alloyed. It is metastable, sensitive to growth conditions, and processed using MBE or MOCVD. Nitride decomposition can release toxic ammonia.
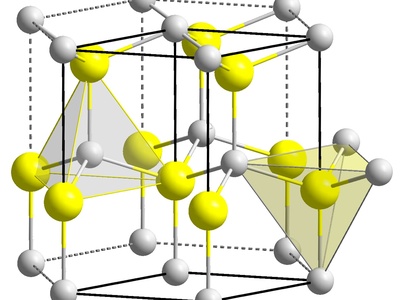
Indium(III) sulfide
A yellow to brown semiconductor used as a pigment and investigated as a light-absorbing layer in thin-film solar cells. Non-toxic relative to cadmium alternatives; multiple polymorphs affect optical properties and processing methods.
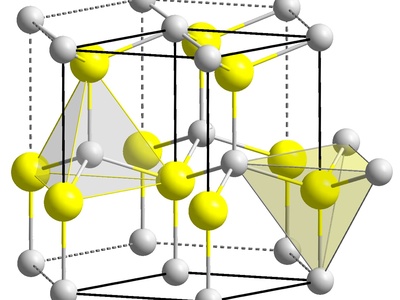
Indium(III) selenide
A layered semiconductor with several polymorphs studied for phase-change memory and photovoltaic applications. Sensitive to stoichiometry and defects; selenides can produce toxic hydrogen selenide when degraded, so manufacturing requires strict controls.
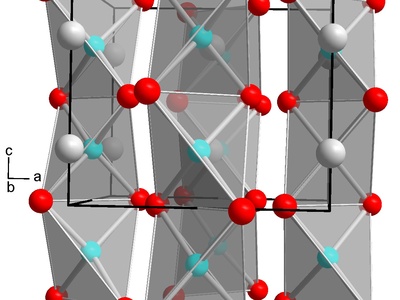
Indium(III) telluride
A semiconductor and thermoelectric material studied for energy conversion; often nonstoichiometric and sensitive to composition. Tellurides may release toxic hydrogen telluride upon decomposition; handled with care in synthesis and device fabrication.
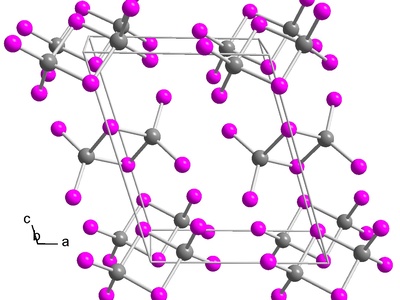
Indium(I) iodide
A grey crystalline compound containing indium in the +1 state, used in specialty inorganic synthesis and some metallurgical processes. Less stable than +3 salts and prone to disproportionation; handled under inert atmosphere to avoid degradation.

Indium(I) chloride
A subhalide often observed in vapor-phase or low-temperature chemistry, featuring indium in +1 oxidation state. Unstable at room temperature and disproportionates to In metal and InCl3; primarily of interest in research rather than industrial use.

Indium monoselenide
A van der Waals layered semiconductor with interesting optoelectronic properties for photodetectors and electronics. Exhibits multiple polytypes and varying oxidation states; sensitive to air and moisture, requiring encapsulation for stable device performance.

Indium monosulfide
A layered or chain-like semiconductor investigated for optoelectronic and photovoltaic applications. Stoichiometry and phase determine properties; can oxidize in air and yields sulfur-containing gases on degradation, so material handling and encapsulation are important.
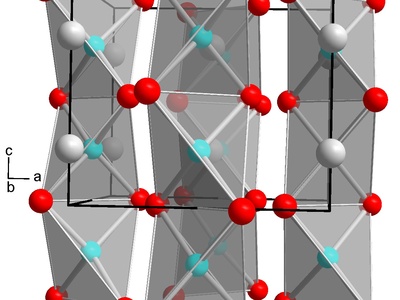
Indium telluride
A layered semiconductor with mixed-valence indium, studied for thermoelectric and electronic applications. Nonstoichiometry and polymorphism influence transport properties; telluride chemistry requires care because of toxic volatile tellurium species during handling.

Indium oxyhydroxide
An oxyhydroxide phase encountered in hydrolysis and precursor routes to indium oxide catalysts and supports. Often amorphous or poorly crystalline; converts to In2O3 on heating. Dusts can irritate lungs and require dust-control measures.

Indium triflate
A soluble, non-coordinating indium salt used as a Lewis acid catalyst in organic synthesis and as a precursor in materials chemistry. Expensive and moisture-sensitive; handled with standard precautions for strong Lewis acids and fluorinated anions.

Indium perchlorate
A strong oxidizing indium salt used mainly in research; perchlorate salts pose explosion and fire hazards, especially when dry. Corrosive and potentially hazardous; typically avoided except when required for specific oxidative or coordination chemistry studies.

Indium oxalate
An insoluble coordination compound used as a precursor for producing indium oxide and mixed-metal oxides by thermal decomposition. Organic anion decomposition releases gases; material handling requires ventilation and care to control dust and decomposition products.