In labs, lectures, and code, a short, reliable list of physical constants makes routine work faster and more accurate. Whether you’re checking a value for a homework problem or building a simulation, having everything in one place reduces second-guessing and mistakes.
There are 27 Atomic and Nuclear Constants, ranging from Atomic mass constant to Vacuum permittivity. For each, you’ll find below the Symbol,Value,Units along with a concise numeric value and unit formatting so you can copy or compare quickly — you’ll find below the full table for easy reference.
How current are these constants and where do the values come from?
Most entries are drawn from recognized compilations (for example CODATA recommendations and standard physics references); check the table notes or source line for the exact revision year. When precision matters, confirm the citation and use the most recent recommended values for high-accuracy work.
Can I use these numbers directly in calculations and simulations?
Yes, but pay attention to units and significant figures: use the listed units exactly (or convert consistently) and consider reported uncertainties when propagating errors. For simulations, prefer double precision and document which constant set you used so results stay reproducible.
Atomic and Nuclear Constants
| Name | Symbol | Value | Units |
|---|---|---|---|
| Speed of light | c | 299,792,458 (exact) | m s^-1 |
| Planck constant | h | 6.62607015e-34 (exact) | J s |
| Reduced Planck constant | ħ | 1.0545718176461565e-34 | J s |
| Elementary charge | e | 1.602176634e-19 (exact) | C |
| Electron mass | m_e | 9.1093837015(28)e-31 | kg |
| Proton mass | m_p | 1.67262192369(51)e-27 | kg |
| Neutron mass | m_n | 1.67492749804(95)e-27 | kg |
| Atomic mass constant | m_u | 1.66053906660e-27 (exact) | kg |
| Avogadro constant | N_A | 6.02214076e23 (exact) | mol^-1 |
| Boltzmann constant | k_B | 1.380649e-23 (exact) | J K^-1 |
| Fine-structure constant | α | 7.2973525693(11)e-3 | dimensionless |
| Rydberg constant | R_∞ | 10,973,731.568160(21) | m^-1 |
| Bohr radius | a_0 | 5.29177210903(80)e-11 | m |
| Hartree energy | E_h | 4.3597447222071(85)e-18 | J |
| Electron volt | eV | 1.602176634e-19 (exact) | J |
| Classical electron radius | r_e | 2.8179403262(13)e-15 | m |
| Reduced Compton wavelength (electron) | λ̄_C | 3.8615926764(11)e-13 | m |
| Bohr magneton | μ_B | 9.2740100783(28)e-24 | J T^-1 |
| Nuclear magneton | μ_N | 5.0507837461(27)e-27 | J T^-1 |
| Vacuum permittivity | ε_0 | 8.8541878128(13)e-12 | F m^-1 |
| Coulomb constant | k_e | 8.9875517923(14)e9 | N m^2 C^-2 |
| Electron-proton mass ratio | m_e/m_p | 5.4461702133(10)e-4 | dimensionless |
| Rydberg energy | R_∞hc | 13.605693009(84) | eV |
| Bohr frequency unit (Hz per Hartree) | E_h/h | 6.579683920721(13)e15 | Hz |
| Atomic unit of length (a.u.) | a.u. | 5.29177210903(80)e-11 | m |
| Atomic mass unit energy equivalent | 1 u c^2 | 931.49410242(28)e6 | eV |
| Classical electron cross-section (Thomson) | σ_T | 6.6524587321(25)e-29 | m^2 |
Images and Descriptions

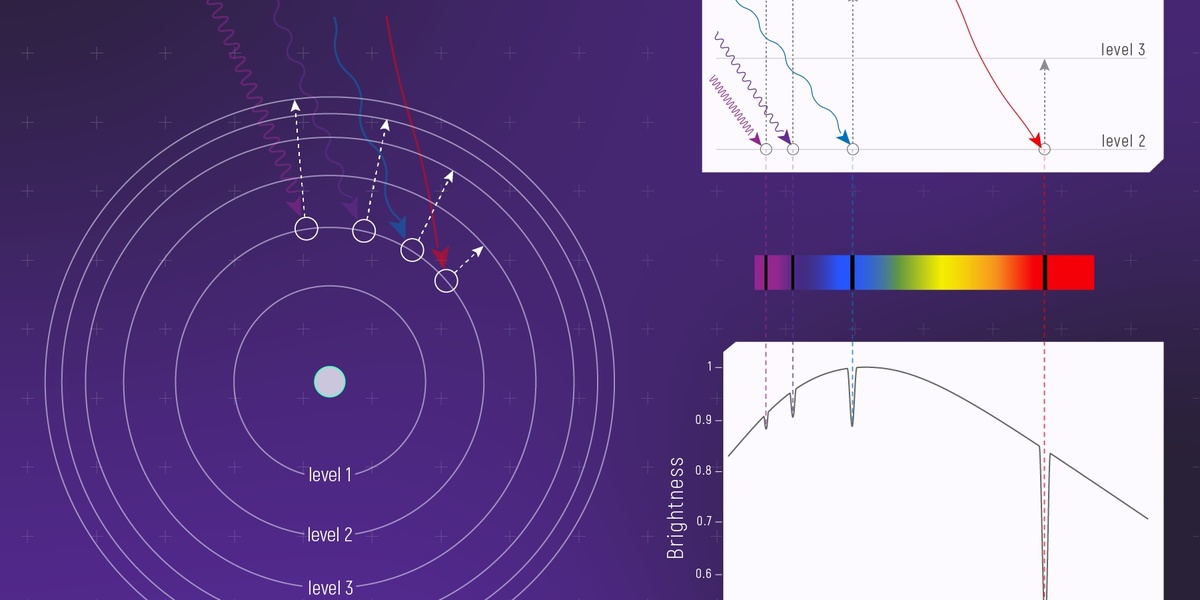
Speed of light
The universal speed limit for massless particles and electromagnetic waves. It links space and time in relativity and converts between mass and energy; central to atomic and nuclear calculations involving photon energies and kinematics.
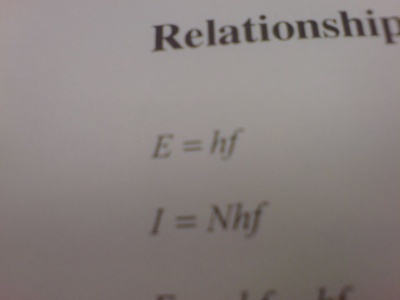
Planck constant
Sets the quantum scale by relating photon energy to frequency. Essential for quantization of energy, spectroscopic transitions, and converting between frequency and energy in both atomic and nuclear physics.

Reduced Planck constant
ħ = h/2π appears in quantum mechanics formulas for angular momentum, commutation relations, and wave mechanics. It’s used to express energies, action, and uncertainty relations common to atomic and nuclear problems.

Elementary charge
The magnitude of the electric charge carried by a proton. Fundamental to electromagnetic interactions, it converts between electrical units and energy (eV) and appears in Coulomb forces for atoms and nuclei.
Electron mass
The rest mass of the electron, critical for atomic structure, spectroscopy, and scattering calculations. It sets electron kinetic energies, wavelengths, and appears in reduced-mass corrections in few-body nuclear problems.

Proton mass
The rest mass of the proton, a primary mass scale in nuclear physics. It matters for nuclear binding energies, mass‑energy conversions, and atomic mass calculations when comparing isotopes.
Neutron mass
The rest mass of the neutron, central to nuclear stability, decay, and reaction energetics. Differences between neutron and proton masses affect beta decay thresholds and isotopic mass balances.
Atomic mass constant
Defined as 1/ N_A kilogram per mole, it provides the unified atomic mass unit (u). Widely used to express nuclear and atomic masses, binding energies per nucleon, and mass defect calculations.

Avogadro constant
Number of entities per mole, linking microscopic particle counts to macroscopic amounts. Used to convert atomic-scale masses to grams and to relate atomic mass units to kilograms in nuclear mass tables.

Boltzmann constant
Connects temperature to energy, allowing thermal distributions of atoms and nuclei to be expressed in energy units. Key for statistical mechanics, reaction rates, and population of excited states.

Fine-structure constant
A dimensionless measure of electromagnetic interaction strength. It controls atomic spectral splittings, QED corrections, and influences precision tests in both atomic and nuclear electromagnetic processes.

Rydberg constant
Gives the wave-number of hydrogenic spectral lines in the infinite-mass limit. Fundamental for atomic spectroscopy, calibrating energy levels, and converting observed wavelengths to atomic energies.

Bohr radius
Typical size of the hydrogen atom, determined by quantum mechanics and electromagnetism. Serves as a length unit in atomic calculations and as a scale for electron orbitals and charge distributions.
Hartree energy
The atomic unit of energy (twice the Rydberg), sets the natural energy scale for electrons in atoms and molecules. Useful for atomic-structure calculations and comparing electronic versus nuclear energy scales.

Electron volt
A convenient energy unit equal to the charge of an electron times one volt. Commonly used in atomic and nuclear physics to express particle energies, transition energies, and decay Q‑values.

Classical electron radius
A classical length scale combining e, m_e and c that describes low-energy electron scattering and radiative processes. Useful as an order-of-magnitude size in atomic-scale electromagnetic interactions.

Reduced Compton wavelength (electron)
The reduced Compton wavelength ħ/(m_e c) marks the quantum-relativistic length scale for electrons. Appears in scattering, quantum field corrections, and links particle mass to wavelength.
Bohr magneton
The magnetic moment scale for electrons due to orbital or spin motion. Key in atomic magnetic resonance, Zeeman splitting, and precision measurements testing quantum electrodynamics.
Nuclear magneton
The magnetic moment unit for nucleons, based on proton mass. Used to express nuclear magnetic dipole moments, hyperfine interactions, and to compare nuclear and electronic magnetic scales.

Vacuum permittivity
Sets the scale for electric fields in vacuum and appears in Coulomb’s law expressed with ε0. Important for calculating electromagnetic forces and potentials in atomic and nuclear contexts.

Coulomb constant
Equal to 1/(4πε0), it quantifies the strength of the electrostatic force. Directly used in Coulomb potentials for electrons in atoms and in modeling repulsion between charged nuclear particles.

Electron-proton mass ratio
A small dimensionless ratio that affects hydrogenic energy levels, reduced-mass corrections, and precision spectroscopy comparing atomic and nuclear mass-related effects.

Rydberg energy
The ionization energy of hydrogen in the infinite-mass limit, widely used as a reference energy for atomic transitions and to compare binding energies across atoms and ions.

Bohr frequency unit (Hz per Hartree)
Converts atomic energy units to oscillation frequencies. Useful when translating computed electronic energies into spectroscopic frequencies or photon energies in atomic and nuclear studies.
Atomic unit of length (a.u.)
The atomic unit of length equals the Bohr radius, used as a natural length scale in atomic calculations and often in nuclear-electronic interaction modeling for compact notation.
Atomic mass unit energy equivalent
Gives the energy equivalent of one unified atomic mass unit in MeV, central for converting between mass defects and nuclear binding energies in nuclear physics.

Classical electron cross-section (Thomson)
The low-energy limit for photon scattering off free electrons. Used as a benchmark in radiation physics, opacity calculations, and some electron scattering approximations in atomic and nuclear contexts.