When exploring actinide chemistry, the compounds of berkelium show how synthetic heavy elements behave in small, well-defined sets. Researchers and students often look to lists to compare composition, oxidation state and the history of each compound’s preparation.
There are 14 Berkelium Compounds, ranging from Berkelium nitride to Tris(cyclopentadienyl)berkelium(III). For each entry you’ll find below Formula,Oxidation state,Synthesis (first report), giving a quick snapshot of composition, typical valence and where the compound was first described—you’ll find below.
How stable are berkelium compounds and how are they handled safely?
Berkelium is radioactive and typically available only in microgram to milligram amounts; its compounds are chemically similar to other late actinides but require specialized radiochemical labs, remote handling, and strict containment to limit alpha and low-energy beta radiation exposure and contamination.
How are those compounds typically synthesized and identified?
Synthesis usually involves small-scale solid-state reactions, metathesis, or organometallic routes under inert atmosphere, and identification relies on X-ray diffraction, spectroscopy (IR, NMR when possible), and radiochemical techniques to confirm composition and oxidation state.
Berkelium Compounds
| Name | Formula | Oxidation state | Synthesis (first report) |
|---|---|---|---|
| Berkelium(IV) oxide | BkO₂ | 4 | Ignition of berkelium ions on a grid in air; 1960s |
| Berkelium(III) oxide | Bk₂O₃ | 3 | Reduction of BkO₂ with hydrogen gas at high temperature; 1960s |
| Berkelium(IV) fluoride | BkF₄ | 4 | Treatment of BkF₃ with elemental fluorine gas at high temperature; 1967 |
| Berkelium(III) fluoride | BkF₃ | 3 | Precipitation from berkelium(III) solution with hydrofluoric acid; 1960s |
| Berkelium(III) chloride | BkCl₃ | 3 | Reaction of berkelium oxide with hydrogen chloride gas at ~500°C; 1962 |
| Berkelium(III) bromide | BkBr₃ | 3 | Reaction of berkelium oxide with hydrogen bromide gas at ~700°C; 1970 |
| Berkelium(III) iodide | BkI₃ | 3 | Reaction of berkelium oxide with hydrogen iodide gas at ~650°C; 1970s |
| Berkelium(III) oxychloride | BkOCl | 3 | Hydrolysis of BkCl₃ with water vapor at high temperature; 1960s |
| Berkelium nitride | BkN | 3 | Reaction of berkelium metal with nitrogen gas at high temperature; 1973 |
| Tris(cyclopentadienyl)berkelium(III) | Bk(C₅H₅)₃ | 3 | Reaction of BkCl₃ with beryllium dicyclopentadienide in a melt; 1970 |
| Dicesium hexachloroberkelate(IV) | Cs₂BkCl₆ | 4 | Oxidation of Bk(III) in concentrated HCl with CsCl present; 1970s |
| Berkelium(III) phosphate | BkPO₄ | 3 | High-temperature reaction of berkelium oxide and ammonium phosphate; 1980s |
| Berkelium(III) sulfate | Bk₂(SO₄)₃ | 3 | Evaporation of a berkelium(III) solution in sulfuric acid |
| Berkelium(IV) iodate | Bk(IO₃)₄ | 4 | Precipitation from a Bk(IV) solution with iodic acid; 1970s |
Images and Descriptions

Berkelium(IV) oxide
A dark brown-black solid, this is the most stable oxide of berkelium. It adopts the common fluorite crystal structure, and its formation is a key step in the purification and storage of berkelium.

Berkelium(III) oxide
A yellowish-green solid, this is the lower oxide of berkelium. It exists in several crystal forms depending on temperature and serves as a common starting material for synthesizing other berkelium(III) compounds.

Berkelium(IV) fluoride
A yellowish-green solid, this compound represents berkelium in its less common but stable +4 state. Its synthesis confirmed the accessibility of tetravalent berkelium, paralleling its neighbor element, terbium, in the lanthanide series.
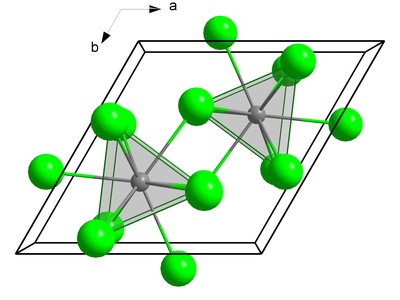
Berkelium(III) fluoride
A yellowish-green solid that is insoluble in water. Its precipitation is a crucial step in the purification and separation of berkelium from other elements during the processing of irradiated nuclear targets at facilities like ORNL.
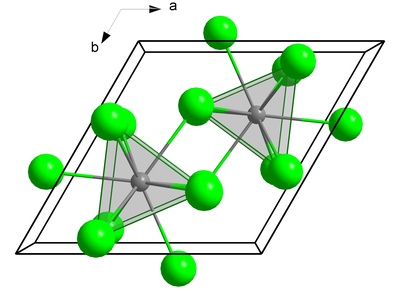
Berkelium(III) chloride
A green, water-soluble crystalline solid that visibly glows red in the dark due to its intense radioactivity. Its structure and properties have been studied to understand chemical trends across the actinide series.

Berkelium(III) bromide
A yellowish-green solid whose crystal structure depends on its preparation method. Studies of its light absorption spectrum provided early insights into the complex electronic structure of the berkelium(III) ion.

Berkelium(III) iodide
A yellow solid, it is one of the heavier halides of berkelium. Its properties are compared with other actinide triiodides to understand periodic trends in bonding and structure for these highly radioactive elements.

Berkelium(III) oxychloride
A solid compound often formed as an intermediate in high-temperature synthesis. It has a layered crystal structure typical of lanthanide and actinide oxyhalides, making it interesting for structural chemistry comparisons.

Berkelium nitride
A black, rock-salt structured solid. As a pnictide (a compound with a group 15 element), it is studied for its potential magnetic and electronic properties, part of a series of actinide nitrides investigated for fundamental physics.

Tris(cyclopentadienyl)berkelium(III)
An amber-colored solid, this was one of the first organometallic compounds of berkelium ever made. Its synthesis proved that berkelium could form bonds with carbon rings, greatly expanding its known chemistry beyond simple salts.

Dicesium hexachloroberkelate(IV)
An orange-yellow crystalline complex salt. This compound is significant as it provides a way to stabilize the rare +4 oxidation state of berkelium in a solid form, allowing for detailed spectroscopic and magnetic studies.

Berkelium(III) phosphate
A solid compound with a highly stable crystal lattice. Like other actinide phosphates, it is of interest for its potential role in the long-term immobilization of nuclear waste due to its exceptional thermal and chemical durability.

Berkelium(III) sulfate
A solid compound studied for its coordination chemistry in aqueous solutions. Understanding its behavior is important for the separation science of actinides, particularly in acidic processing streams used in nuclear fuel reprocessing.

Berkelium(IV) iodate
A solid compound prepared by precipitating berkelium from a solution where it was stabilized in its +4 oxidation state. Its low solubility is characteristic of tetravalent actinide iodates, a property useful in analytical chemistry.